Heart failure and Calcium regulation
A number of cardiac abnormalities are associated with advanced heart failure (HF), all leading to a reduced ability of the heart to pump blood and supply adequate oxygenated blood to the body. Reduced contractility of the individual cardiomyocytes is the main factor resulting in reduced global cardiac output, and intracellular calcium (Ca2+) flux is central to this cellular abnormality.
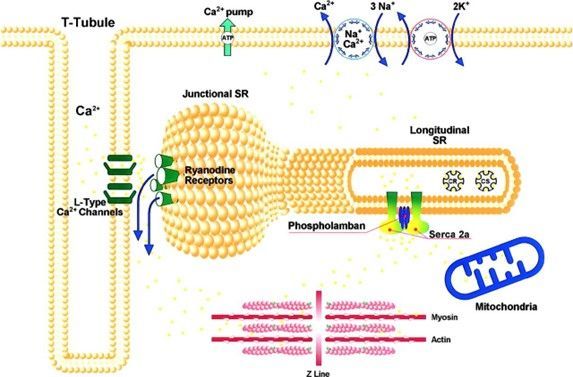
Regulation of excitation–contraction coupling in cardiomyocytes is driven by Ca2+ flux between the sarcoplasmic reticulum (SR) and the cytosol. Electrical excitation of the sarcolemma leads to the opening of voltage gated L-type Ca2+ channels, allowing the entry of a small amount of Ca2+ into the cell (Figure and video). This influx leads to opening of the ryanodine receptors located on the SR membrane and a large amount of Ca2+ is released from the SR into the cytosol. The resulting increase in cytosolic Ca2+ activates the myofilaments resulting in contraction. [1, 2] During relaxation, Ca2+ is pumped back into the SR by the SR Ca2+ ATPase (SERCA2a) pump and extruded extracellularly by the sarcolemmal Na+/Ca2+ exchanger. The contribution of SERCA2a in humans for removing intracellular calcium is ~75% and ~25% for the Na+/Ca2+ exchanger. The Ca2+ pumping activity of SERCA2a is regulated by phospholamban. In the unphosphorylated state, phospholamban inhibits the Ca2+-ATPase, whereas phosphorylation of phospholamban by cAMP-dependent protein kinase and by Ca2+-calmodulin dependent protein kinase releases the inhibition.
SERCA2a, a proven effector crucial for Calcium Regulation, is downregulated in heart failure
In the human heart, intracellular Ca2+ movements are tightly regulated at various levels. As the key effector in cardiac Ca2+ homeostasis or regulation, SERCA2a plays a central role in regulating contraction and relaxation by its role in controlling the level of Ca2+ in the cytosol and SR in the cardiomyocyte. [3]
Defects in Excitation-Contraction coupling, due to abnormal expression and/or function of calcium handling and transport proteins, are a hallmark of cardiac dysfunction. These defects manifest in changes in the calcium transient: reduced amplitude, increased duration, and prolonged decay – the consequence of which is decreased contractility and reduced cardiac output. Regardless of the etiology of heart failure, the role of reduced SR calcium load has been well established in terms decreased SR calcium uptake, decreased SR calcium content, and calcium leak from the SR. [4] [5] This is primarily due to a decrease in SR calcium uptake because of SERCA2a dysfunction. In both animal models of and patients with heart failure, there is a reduction in SERCA2a expression (mRNA and protein levels) and activity. [6]
SERCA2a as a validated target for the treatment of heart failure
Increasing the levels of SERCA2a protein in cardiomyocytes has been shown in human cells, experimental rodent and large animal models to normalize the abnormally high diastolic levels of cytosolic calcium typical of HF and improve contractile and clinical outcomes, as described below. [7-9] [10-13]
SARDOCOR’s founder, President and Chief Medical Officer, Dr. Roger Hajjar, is an absolute international pioneer and leader in Calcium regulation and Heart Failure. With over 500 peer-reviewed publications in numerous first-rated journals such as Nature, Nature Biotechnology, Nature Communications, Circulation, Circulation Research, etc, Dr. Hajjar started working on SERCA2a biology over three decades at the Massachusetts General Hospital at Harvard and has been developing translational technologies to benefit heart failure patients. Based on Dr. Hajjar’s ground-breaking research, SARDOCOR is using gene transfer as a method to restore SERCA2a function in heart failure patients using a recombinant adeno-associated viral vector (AAV), which consists of an AAV serotype 1 capsid and contains the human SERCA2a cDNA flanked by ITRs derived from AAV serotype 2 (AAV1/SERCA2a). Indeed, various academic and industrial groups are similarly developing SERCA2a activators for the treatment of heart failure (e.g., i1c, DWORF, small molecules).
Dr. Hajjar’s technologies have been previously licensed to Celladon, Nanocor, Bayer’s Askbio, etc. Even with the correct molecular target, the dosage of AAV needs to be within the right range for any biological effects to kick in. Sardocor’s technologies are unique by targeting a proven effector and by titrating the best dosages with the use of Novoheart’s proprietary, world’s first human mini-heart technology (www.novoheart.com) in accordance with the US FDA’s Modernization Act 2.0. for human relevance and accuracy without depending on animals. Taken together with Sardocor’s proprietary heart-specific intracoronary delivery method, the combined scientific approach aims to reduce side effects (such as immune responses) while achieving efficacy.
In addition to the role in cardiomyocytes, SERCA2a also has a role in skeletal muscle, the diaphragm, vascular smooth muscle, and endothelial cells. Both moderate and high intensity exercise increase the relative SERCA2a isoform expression in cardiac and skeletal muscle cells. SERCA2a gene transfer also results in increased coronary blood flow in a diabetic rat model. Finally, increasing SERCA2a levels via gene transfer inhibits vascular smooth muscle proliferation.
References:
- Bers, D., Cardiac excitation-contraction coupling. Nature, 2002. 415: p. 198- 205.
- Bers, D., Calcium fluxes involved in control of cardiac myocyte contraction. Circulation Research, 2000. 87(4): p. 275-81.
- Bers, D., Sarcoplasmic reticulum Ca release in intact ventricular myocytes. Frontiers in Bioscience, 2002. 7: p. d1697-711.
- Kranias, E.G. and R.J. Hajjar, Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res, 2012. 110(12): p. 1646-60.
- Gorski, P.A., D.K. Ceholski, and R.J. Hajjar, Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab, 2015. 21(2): p. 183-194.
- Meyer, M., et al., Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation, 1995. 92(4): p. 778-84.
- del Monte, F., et al., Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation, 1999. 100(23): p. 2308-11.
- del Monte, F., et al., Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation, 2001. 104(12): p. 1424-9.
- Miyamoto, M.I., et al., Adenoviral gene transfer of SERCA2a improves left- ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A, 2000. 97(2): p. 793-8.
- Beeri, R., et al., Gene delivery of sarcoplasmic reticulum calcium ATPase inhibits ventricular remodeling in ischemic mitral regurgitation. Circ Heart Fail, 2010. 3(5): p. 627-34.
- Kawase, Y., et al., Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol, 2008. 51(11): p. 1112-9.
- Prunier, F., et al., Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation, 2008. 118(6): p. 614-24.
- Sakata, S., et al., Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol, 2007. 42(4): p. 852-61.

